Medicare Improperly Paid DMEPOS Suppliers $22.7M
Uncategorized
October 28, 2025
Medicare Improperly Paid DMEPOS Suppliers $22.7M
WASHINGTON—A recent report shows Medicare has continued to improperly pay millions for durable medical equipment, prosthetics, orthotics and supplies (DMEPOS) provided to enrollees during inpatient stays despite a major audit in 2018 and subsequent fix in 2020.
A Look at the Numbers
An audit from the Health and Human Services (HHS) Office of Inspector General (OIG) found that since 2020, Medicare has improperly paid DMEPOS suppliers $4.5 million for inpatient DMEPOS. The full audit found that a total of $22.7 million has been improperly paid since 2018, following its initial audit.
The 2018 OIG audit found that Medicare improperly paid suppliers $34 million from 2015 to 2017. The improper payments occurred due to inadequate system edits that should have prevented or detected the overpayments. OIG recommended that the Centers for Medicare & Medicaid Services (CMS) correct the system edits to fully prevent and detect overpayments to suppliers for DMEPOS items provided during inpatient stays. CMS concurred with the OIG’s recommendation, and in January 2020, CMS modified its Common Working File (CWF) for verification, validation and payment authorization—a claims processing system—to detect and prevent those claims.
Inpatient facilities receive Medicare Part A payments in full for all services provided, including DMEPOS items. Therefore, Medicare Part B should not cover a supplier for DME that is given to an enrollee when they are still inpatient.
Since the prior audit found a large overpayment, the OIG conducted a follow-up audit to determine whether payments met requirements or not. Following the initial audit from 2018 to 2019, $18.2 million was improperly paid to suppliers. After the CWF was edited in January 2020, $4.5 million has been improperly paid to DMEPOS suppliers for items provided during inpatient stays through December 2024.
In addition to the total $22.7 million in improper payments from 2018 to 2024, the OIG found that enrollees may have been held responsible for unnecessary deductibles and coinsurance of up to $5.9 million paid to suppliers for the items.
The OIG recommended that CMS review the CWF system edits to identify if any refinements could be made to prevent further improper payments to suppliers. However, CMS said the initial edit significantly reduced improper payments and that it reviewed a sample of claims by OIG and could not identify a systematic cause for the errors. CMS also said that the Recovery Audit Contractor (RAC) has an approved automated review, and some of the claims identified by the OIG may have already been addressed by the RAC review.
“We acknowledge the actions that CMS has taken,” the OIG said. “… We maintain that our recommendation is valid. We found that improper payments of $4.5 million were still made from January 2020 through December 2024, even after the CWF edits were modified.”
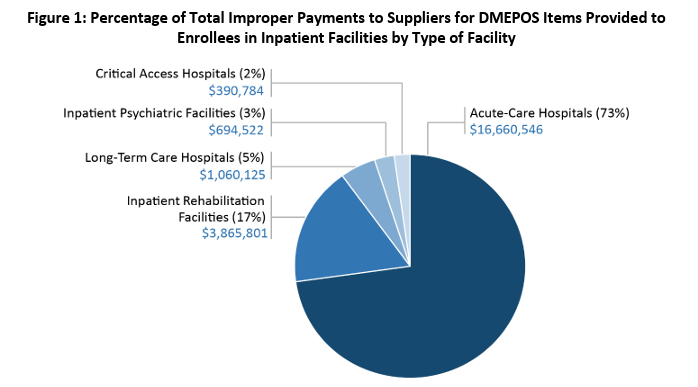
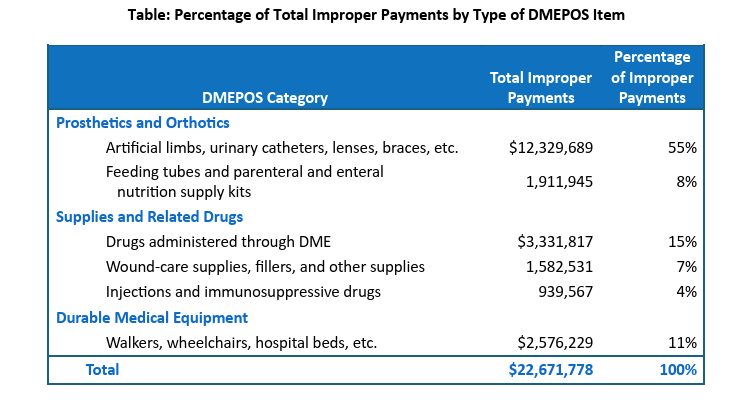
OIG Recommendations
The OIG said edits CMS made following the initial audit were effective in reducing improper payments, however, further refinements may be necessary. The OIG recommended that CMS:
- Direct the durable medical equipment Medicare administrative contractors (DME MACs) to recover from suppliers up to $22.7 million in identified improper payments for our audit period that are within the four-year reopening period in accordance with CMS’s policies and procedures.
- Direct DME MACs to recommend that the suppliers refund to enrollees up to millions in deductible and coinsurance amounts that may have been incorrectly collected from them or from someone on their behalf.
- Instruct the DME MACs to notify, as CMS deems appropriate, suppliers that received an overpayment or overpayments to consider conducting one or more internal audits or investigations based on the risks identified by this audit to identify any similar overpayments the supplier might have received and return any identified overpayments to the Medicare program.
- Identify any DMEPOS claims after the OIG’s audit period for items provided to enrollees during inpatient stays and direct the DME MACs to recover any improper payments.
- Review system edits to determine whether any refinements are necessary to prevent improper payments to suppliers for DMEPOS items provided to enrollees during inpatient stays.
CMS Comments
CMS concurred with the first and fourth recommendations and explained actions it had taken and planned to take to address them. CMS did not concur with the fifth recommendation. The OIG said it still maintains that the fifth recommendation is valid.
For OIG’s full report, visit here.

